1. Molecular mechanisms of ion channel activation
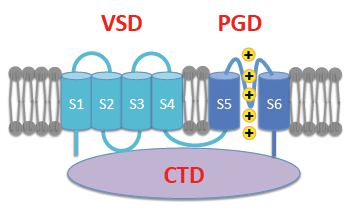 Many ion channels are formed by structurally distinct sensory and pore-gate domains (PGD). These structural domains have different origins and have come together during evolution to combine sensitivity to various cellular signals with conductance to specific ions. For instance, the PGD structure is conserved among K+, Na+, and Ca2+ selective channels, while a voltage sensing domain (VSD) is found in many types of voltage-sensitive ion channels as well as in voltage-sensing lipid phosphatases. A fundamental question is how these structural domains are coupled and interact such that conformational changes in the sensing domain, in response to physiological stimulation, are propagated to the PGD to regulate channel opening?
Many ion channels are formed by structurally distinct sensory and pore-gate domains (PGD). These structural domains have different origins and have come together during evolution to combine sensitivity to various cellular signals with conductance to specific ions. For instance, the PGD structure is conserved among K+, Na+, and Ca2+ selective channels, while a voltage sensing domain (VSD) is found in many types of voltage-sensitive ion channels as well as in voltage-sensing lipid phosphatases. A fundamental question is how these structural domains are coupled and interact such that conformational changes in the sensing domain, in response to physiological stimulation, are propagated to the PGD to regulate channel opening?
To answer this question, we study BK type K+ channels. BK channels are activated by membrane voltage, and binding of intracellular Ca2+ and Mg2+. The channel protein consists of a pore-gate domain (PGD), four voltage sensing domains (VSD), and a large cytosolic domain (CTD) that binds to intracellular divalent cations, thus providing an excellent model system to study the functional coupling among these modular domains. We use patch clamp techniques to record VSD movements and PGD opening; mutagenesis, chemical modification and protein biochemistry to detect domain-domain interactions; and molecular and thermodynamic modeling to predict and interpret experimental results.
As membrane proteins, ion channels have evolved in the context of lipids. In addition to the protein-protein interactions, we also study if and how lipid molecules are involved in the coupling between modular channel domains. This study focuses on the modulation of KCNQ K+ channels by phosphatidylinositol 4,5-bisphosphate (PIP2), a rare phospholipid found exclusively in the inner leaflet of the plasma membrane. In addition to the approaches described above, we also use voltage clamp fluorometry to monitor VSD conformation in real time.
2. Ion channels in association with diseases
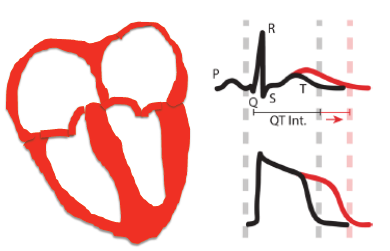 BK channels play important roles in neural transmission and smooth muscle contraction, while KCNQ channels are important in maintaining normal heart rhythm and neuronal excitability. We study modulation of these channels by cellular signaling pathways and mutations in association with physiology and pathophysiology in nervous system and heart.
BK channels play important roles in neural transmission and smooth muscle contraction, while KCNQ channels are important in maintaining normal heart rhythm and neuronal excitability. We study modulation of these channels by cellular signaling pathways and mutations in association with physiology and pathophysiology in nervous system and heart.
Arrhythmia and sudden cardiac death often accompany ischemia. During ischemia, reduced oxygen and glucose supply to myocytes results in abnormal metabolism and lower ATP production. Understanding how these complex changes induce arrhythmia is essential for developing effective anti-arrhythmic strategy. We are studying ATP binding and activation of KCNQ1 channels, which provides a direct link between cellular metabolism state to electrical properties of the cell membrane. By experiments on the KCNQ1 molecules and in cardiac myocytes we expect to reveal a novel mechanism for ischemia induced arrhythmia.
Mutations of BK or KCNQ channels, or their associated proteins, have been linked to various diseases (channelopathies) including cardiac arrhythmia, epilepsy and abnormal blood pressure. However, for many of these mutations how they affect channel function and thereby the pathogenesis of diseases is not clear. We try to answer this question based on our studies of the fundamental mechanisms of ion channel activation. For example, we discovered that a mutation in KCNQ1 and a number of mutations in a KCNQ1 associated protein, KCNE1, linked to cardiac arrhythmia by disrupting VSD movement and reducing PIP2 sensitivity, respectively. We have also been studying how an epilepsy-linked BK channel mutation strengthens the coupling between Ca2+ binding domain and the pore-gate domain. The study of these naturally occuring mutations also provides fundamental insights into the basic mechanisms of ion channel activation.
3. Discovery of channel-modifying compounds
Applying our mechanistic understanding of ion channel activation, we search for small molecules and animal toxins (such as peptides from snake and scorpion venoms) that modify channel function. These ion channel modifiers can serve as lead compounds for drug development or be used as research tools to study ion channel structure, function and physiological roles in vitro and in vivo. For example, we have used computational methods to screen a library of more than 30,000 small molecules against a focal point in the structural model of the KCNQ1 channel. This focal point has been identified in our previous studies as being critical for voltage dependent activation of the channel. The in silico screen has resulted in about 40 positive hits, and subsequent experimental studies confirmed that 3 of these chemicals modify channel function. Further experiments are now under way to elucidate the molecular mechanism of how these chemicals alter channel function.
 Many ion channels are formed by structurally distinct sensory and pore-gate domains (PGD). These structural domains have different origins and have come together during evolution to combine sensitivity to various cellular signals with conductance to specific ions. For instance, the PGD structure is conserved among K+, Na+, and Ca2+ selective channels, while a voltage sensing domain (VSD) is found in many types of voltage-sensitive ion channels as well as in voltage-sensing lipid phosphatases. A fundamental question is how these structural domains are coupled and interact such that conformational changes in the sensing domain, in response to physiological stimulation, are propagated to the PGD to regulate channel opening?
Many ion channels are formed by structurally distinct sensory and pore-gate domains (PGD). These structural domains have different origins and have come together during evolution to combine sensitivity to various cellular signals with conductance to specific ions. For instance, the PGD structure is conserved among K+, Na+, and Ca2+ selective channels, while a voltage sensing domain (VSD) is found in many types of voltage-sensitive ion channels as well as in voltage-sensing lipid phosphatases. A fundamental question is how these structural domains are coupled and interact such that conformational changes in the sensing domain, in response to physiological stimulation, are propagated to the PGD to regulate channel opening? BK channels play important roles in neural transmission and smooth muscle contraction, while KCNQ channels are important in maintaining normal heart rhythm and neuronal excitability. We study modulation of these channels by cellular signaling pathways and mutations in association with physiology and pathophysiology in nervous system and heart.
BK channels play important roles in neural transmission and smooth muscle contraction, while KCNQ channels are important in maintaining normal heart rhythm and neuronal excitability. We study modulation of these channels by cellular signaling pathways and mutations in association with physiology and pathophysiology in nervous system and heart.
